Describe Ph Scale Acids and Bases
Values greater than 7 are basic the higher the number the more basic. Easy as 1 2 314.
Buffers Ph Acids And Bases Biology For Non Majors I
Acids are situated below 7 while bases or alkaline substances are found above 7.
. It has more hydroxide ions OH than hydrogen ions H. The more hydrogen ions present in solution the lower the pH of the chemical. Acid a solution that is between 0-6 on the pH Scale.
What are acids and bases. In the middle is the number 7. An Active Learning Approach.
At a pH of 7 a chemical is said to be neutral as equal amounts of. Contains more hydrogen ions H than hydroxide ions OH. The pH scale is used to rank solutions in terms of acidity or basicity alkalinity.
Define and describe the properties of acids and bases with respect to hydrogen ions. PH log 10 H 3 O aq. Bases define indicator A compound that changes color in the.
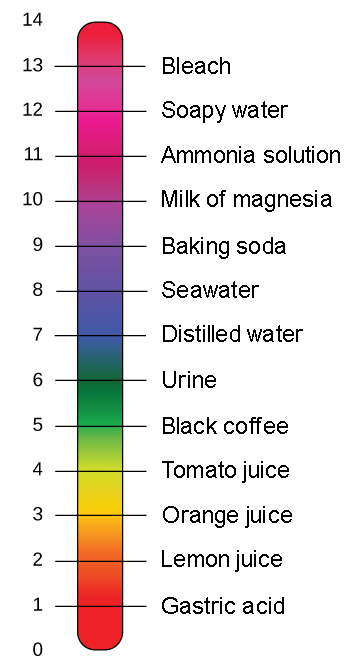
H 2O H 2O H 3O OH. PH scale The average pH of stomach acid Two Neutral substances blood water and oil are examples of __________ Basic substances Toothpaste urine and bleach are examples of ___________ Acidic Substances coffee lemon juice and sweat are examples of __________ Has a PH of 0-69 Acid Has a PH of 71-140 Base Has a PH of 70 Neutral. Most parts of our body excluding things like stomach acid measure around 72 and 76 on the pH scale a 7 is neutral on the scale.
Another way to check if a substance is acidic or basic is to use litmus paper. Describe pH scale buffers acids and bases. The use of scientific notation to describe ion concentrations is somewhat cumbersome and chemists have agreed to employ a pH scale to state the concentration of hydronium ions the capital H in pH stands for hydrogen.
Acids and Bases Acids Bases and the pH Scale 2 H2O 1 H3O 1 OH- We begin with two water molecules and move some hydrogen atoms around. PH values between 1 and 7 indicate an acidic solution whereas pH values between 7 and 14 indicate a basic solution. Above 7 on pH scale.
They define pH to be the negative logarithm to base 10 of the hydronium ion concentration. Strong Acids and Bases. Patient education refers to the process through which information is imparted to the patient and his families by the health care professionals.
Define and draw the pH scale d. Acid added to water This will result in pH values less than 7 Bases added to water. Define buffers and explain how they are related to biological systems.
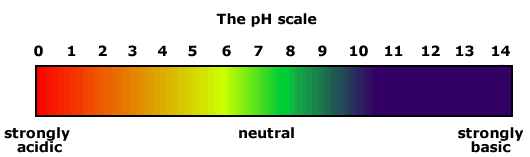
A pH of 7 is neutral. A pH scale measure can vary from 0 to 14 where 0 is the most acidic and 14 is the most basic a substance can be. Since pH -logH the higher the pH the lower the hydrogen ion concentration and vice versa.
From 0 to 7 are acids with 0 being the strongest. Acid Base Properties of Water Water can act as both an acid and a base. The farther from 7 the stronger the acid.
Describe the pH scale. Acid is on one side base is on the other with the pH scale acts as the seesaw board that theyre sitting on. Define and describe acids and bases b.
0-14 7 being neutral. From 7 to 14 are bases with 14 being the strongest base. Base a solution that has a pH from 8-14.
PH scale ranges from. It has an even number of hydrogen ions H and hydroxide ions OH. One water molecule can accept a proton from a second or one water molecule can donate a proton to a second.
Small intestine Describe the pH scale 0-69 are acids 7 are neutral 8-14 are bases stomach fluid lemon juice vinegar HCI and citrus fruit are all Acids ammonia NaOH KOH and soap are. PH is a number from 0 to 14. The pH scale goes on measurements from 0-14.
Explain how neutralization disassociation reactions relate to the formation and neutralization of acids and bases. Describe water as an inorganic compound and universal solvent 7. Step 1 of 5.
Bases added to water Causes the formation of additional hydroxyl ions and a resulting lower concentration of hydronium ions. According to the Arrhenius definition acids are substances that produce hydrogen ion in water while bases are substances that produce hydroxide ion in water. Such an effort by a health care professional helps improve the health status and alter the health behavior of the.
The pH scale is a graphic description of the hydrogen or hydroxide ion present in a sample. If a liquid has a pH of 7 its neutral. This scale shows some representative fluids and their approximate pH values.
Protons belong to the family of particles known as. Scientists use something called a pH scale to measure how acidic or basic a liquid is. Compare and contrast acids and bases c.
The pH scale our seesaw has numbers running across it. Alkaline solutions called bases have a pH higher than 7. Since the scale is based on pH values it is logarithmic meaning that a change of 1 pH unit corresponds to a ten-fold change in H ion concentration.
Below 7 on pH scale. 1 Introduction To Chemistry And Introduction To Active Learning 2 Matter And Energy 3 Measurement And Chemical Calculations 4 Introduction To Gases 5 Atomic Theory. PH -log H Acid added to water Causes the formation of additional hydronium ions and a resulting lower concentration of hydroxyl ions.
The pH scale ranges from 0 to 14. Learn vocabulary terms and more with flashcards games and other study tools. Define the terms salt and electrolyte and give examples of physiological significance.
Its a balancing act between acids and bases. The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is. If foreign strong substances dramatically change this pH our bodies can no longer function properly.
Explain what the pH scale measures and state acidic neutral and basic pH values. This would be something like distilled water. A pH of 7 is perfectly neutral.
Acids have a pH lower than 7. Neutral a solution that has a pH of around 7. Values less than 7 are acidic the lower the number the more acidic.
Acids0-6 Neutral Substance7 Bases8-14 The strongest acid is 0 and as the numbers goes higher 1 2 3 etc the strength of the given. The numbers go from 1 to 2 up to 14. One water molecule gains a hydrogen and therefore takes on a positive charge while the other water molecule loses a hydrogen atom and therefore becomes negatively charged.
Proton A subatomic particle that is one of the basic building blocks of the atoms that make up matter. Explain the function of a buffer. There are two types of litmus paper available that can be used to identify acids and bases red litmus paper and blue litmus paper.
Again the farther above 7 the stronger the base.
Acids And Bases 8 31 The Ph Scale

Comments
Post a Comment